High-performance materials for medical and wearable devices
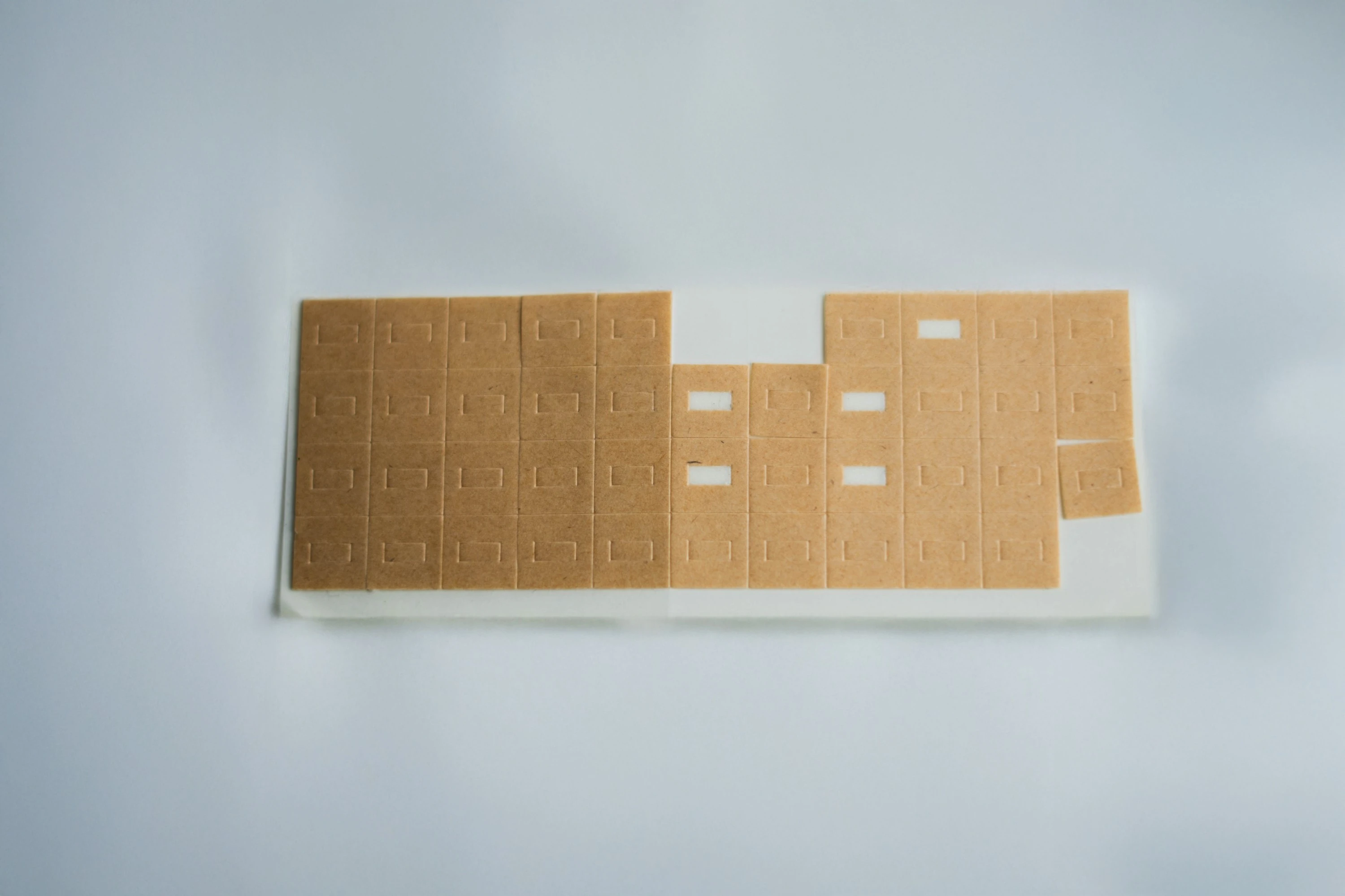
For more than 25 years, Kenosha has supplied medical-grade adhesives, films, and tapes that enable safe, comfortable, and reliable skin contact applications.
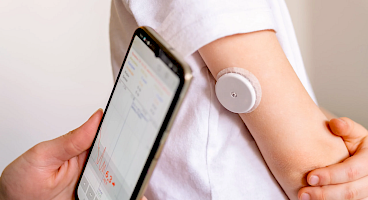
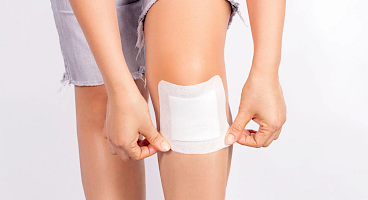
From continuous monitoring sensors and transdermal patches to wound dressings and wearable diagnostics, our materials are engineered to perform. Ensuring comfort, breathability, and adhesion throughout the device’s lifetime.
Kenosha’s materials are biocompatible, traceable, and precision-converted, produced under ISO 9001:2025–certified quality systems to meet the requirements of regulated medical and healthcare manufacturing.